|
Protein Phosphatase Inhibitor-2
(Catalog # P027)
Description:
Protein phosphatase inhibitor-2 specifically
inhibits the catalytic subunit of type 1 protein phosphatases (PP1) at
nanomolar concentrations (IC50 ~ 2 nM). Protein phosphatase inhibitor-2 can
be used to distinguish type 1 protein phosphatases from type 2 protein
phosphatases, which are the major protein serine/threonine phosphatases in
eurkaryotic cells (1, 2). Human recombinant protein phosphatase inhibitor-2 is produced from E. coli., purified from
freshly extracted E. coli lysates, and purified to homogeneity using
methods developed by LAE Biotechnology Co., LTD. The final fraction of protein phosphatase inhibitor-2
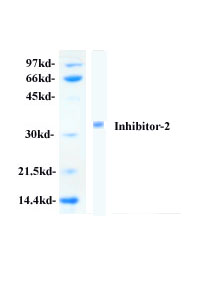
exhibits a single polypeptide band of 31 kDa, which is a heat stable protein
containing 204 amino acid residues.
Quality of Protein Phosphatase Inhibitor-2:
Molecular Weight:
Theoretical: 22,800 daltons and Apparent: 31,000 daltons
1X PP1 Reaction Buffer:
50 mM Tris-HCl, 5 mM dithiothreitol,
0.1 mM EDTA, pH 7.0
Reaction Conditions:
1X PP1 Reaction Buffer supplemented with
1 mM MnCl2 and 5 mM Caffeine
Reaction should be performed at 30 C
Supply:
Protein phosphatase inhibitor-2 is supplied in
lyophilized form at the dose of 100 ug per vail (Catalog # P027)
Reconstitution:
Lyophilized protein phosphatase inhibitor-2 should be should
be reconstituted in 100 ul of 50 mM Tris-HCl, pH 7.0 to make a final
concentration of 1 mg/ml.
Shipping/Storage:
protein phosphatase inhibitor-2 is shipped on ice and must
be stored at -20 C or lower.
Legal consideration: FOR RESEARCH USE ONLY
Notes:
1. Supplement with 1 mM MnCl2 and 5 mM caffeine
(required only if phosphorylase a is the substrate).
2. Protein phosphatase inhibitor-2 has been purified
to > 95% homogeneity as determined by SDS-PAGE and Coomassie Blue staining.
3. Recommended long term storage -70 C; avoid repeated freeze/thaw cycles.
Quality Assurance Statement:
Protein phosphatase inhibitor-2 contains no
detectable protease activity. Tests for phosphatase activity showed no
detectable activity.
References:
1. Ingebritsen, T.S. and Cohen, P. (1983) Eur. J. Biochem., 132, 255-261.
2. Cohen, P. (1991) Methods Enzymol., 201, 389-398.
| 




